Part 1 of the FAQ began an exploration of the first Philips AED, a very innovative personal defibrillator which completely changed the way, place, operation, user role, and time-to-administer electric-shock resuscitation, as well as the popular thinking and expectations about it.
Q: What other challenges did Philips face in delivering the desired BTE waveform?
A: In addition to generate the unusual waveshape, the Philips unit had to drive this bipolar, high-voltage, carefully controlled energy pulse into the body’s impedance from a small, unipolar supply. (For children, the BTE waveform “dose” is far less: up to +600 V and –200 V, spanning 8 to 20 msec, with the energy of 50 joules.)
Q: Is the defibrillation waveform static and unchanging during a resuscitation sequence?
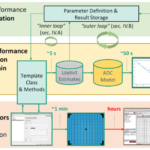
A: No, the actual current and voltage delivered cannot be fixed values which are set in advance. These parameters, and thus the energy delivered, must be adjusted dynamically between during pulses delivery variations in the actual impedance of the patient, which can range from 80 to 90 Ω for adults. To achieve this delivery, Philips adds additional “intelligence” to the biphasic waveform as part of their proprietary “SMART Biphasic” algorithm (Figure 1). The medical and electronic complexities of this implementation of a biphasic waveform as compared to simpler BTE version are explained in detail in Reference 1.
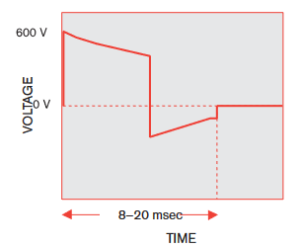
Q: How is the SMART Biphasic waveform defined?
A: It is done by assessing the patient’s impedance to manage the continuous interplay of voltage, current, and energy while delivering the BTE waveform shape. Before the defibrillator generates even that first pulse, it reads the patient’s ECG (electrocardiogram, also spelled EKG due to its German origins as Elektro-kardiographie) to determine if shock is even the appropriate treatment. It also determines the impedance of the patient’s chest by injecting a low-level AC current at the contact electrodes and measuring the resultant voltage drop; impedance is a function of sweat, body size and fat content, and other factors. Only then does it generate a pulse with the appropriate specific BTE waveform values. After each jolt, the impedance is re-measured and the pulse set-up modified as needed (impedance can change during the process due to many factors.)
Q: How was the BTE waveform created?
A: Due to the complexity of the BTE waveform shape, the design team considered using digital synthesis for its generation, but this was rejected due to complexity, cost, and consistency issues associated with system transients. They also looked at using a supercapacitor to store and then release battery energy for this high-rate, high-energy discharge/recharge cycle, but this was ruled out due to size and long-term performance concerns; instead, they used a standard, very high-quality 100-μF film capacitor.
Q: How was a capacitor used to do this?
A: The pulse circuit manages the discharge of this capacitors via a bank of silicon-controlled rectifiers (SCRs) and an IGBT (insulated-gate bipolar transistor) which are, in turn, controlled by the system CPU, which is a custom ASIC. The design needed special attention to controlling the current’s di/dt slewing which occurs as the circuit reverses the waveform polarity via the SCRs to create the biphasic (bipolar) swing. It does so by generating the positive-going waveform, turning the waveform generator off for a few hundred microseconds to allow transients to settle, then generating the negative-going portion.
Q: How was the AED powered?
A: The battery pack was composed of nine standard CR123 lithium batteries rated for nominal 9 V/4.2 AHr capacity which Philips decided to provide as a sealed, custom pack. Although this seems to limit replacement options, it avoided the possibility that users might insert an individual cell incorrectly, use non-lithium cells with the same form factor but different voltage or AHr ratings, or use lower-quality or even counterfeit third-party replacement cells.
Q: How was the core ASIC verified in design?
A: The simulation of the ASIC’s performance during its approximately 1.5-second operating cycle took about 11 days on a high-end PC (remember, this was 2002).
Q: Why not use a display or a basic graphical user interface (GUI) in these first AED units? How was the voice guide for the user created?
A: A GUI would have required more power, design complexity, size, software, and cost. Although generating the guiding voice via computer-voice digital synthesis would have required the least amount of memory, it would have sounded somewhat harsh and artificial (again, it was 2002; things have changed). Instead, there’s a calm, reassuring voice which guides the user (who is very likely in a state of near-panic) was derived from a human narrator who recorded the needed phrases in a relaxed and clear voice. These phrases were digitized, concatenated, decoded, and amplified, to be sent to the unit’s small loudspeaker.
Q: Was there a performance and readiness issue due to the inevitable long periods of AED inactivity and non-use?
A: Yes, absolutely, there was a significant concern with a unit such as this, as it would likely be unused and unchecked for extended periods (unlike a defibrillator in an ambulance or emergency vehicle). To ensure that the battery pack and system were still viable despite months and perhaps years of inactivity, the unit was designed to run daily self-test procedures. These tests assess battery condition, various blocks of the internal circuitry, the overall waveform-generation and delivery functions, the calibration of key components, and the high-voltage circuitry.
Q: How were the prototypes tested and approved?
A: As with any product, but especially one in the medical area with direct life-support consequences, there are two questions: 1) is it working to the target specifications, and b) are those specifications correct? The Philips team answered the first question with extensive tests and measurements using industry-standard patient simulators from Symbio and Dynatech-Nevada (subsequently part of Fluke Biomedical). They answered the second question via a detailed analysis of the extensive array of fully vetted medical literature which reported on tests done on animals, as well as data analyzing the performance of existing non-portable defibrillators.
Q: Is the unit sold today the same as the original unit?
A: Of course, not — but it still is, in many ways. There are several reasons for this. First, as the production matures, costs and uncertainties go down with corresponding volume and “experience.” Second, with any medical product which needs regulatory approval, implementing changes can be difficult, costly, and risky process.
Q: Why is that?
A: First, the vendor has to document the change and state if it is trivial, modest, or major, and then prove that assertion. Next, the vendor must requalify the product, an effort with scope dependent on the classification of the proposed change. Even a seemingly small change, such as going to an improved version of a component which appears to be a “form, fit, and functional” replacement, can trigger a major requalification cycle. Further, the regulatory environment has changed with the introduction of new, more-stringent mandates and rules worldwide.
Q: Have new features and functions been added to the original AED?
A: Yes, new models offer wired and wireless connectivity to download the waveform to a smartphone or PC, for example, as well as more diagnostic and patient reporting, among other features. But the key for a mass-market AED is to keeps the functions and features simple.
Q: Is Philips the only vendor of AEDs?
A: No, other medical-equipment vendors also saw the opportunity. Their ambitions were aided by ICs which implement many of the I/O, management, and control functions, and so greatly simplify party of the design task, (Figure 2).
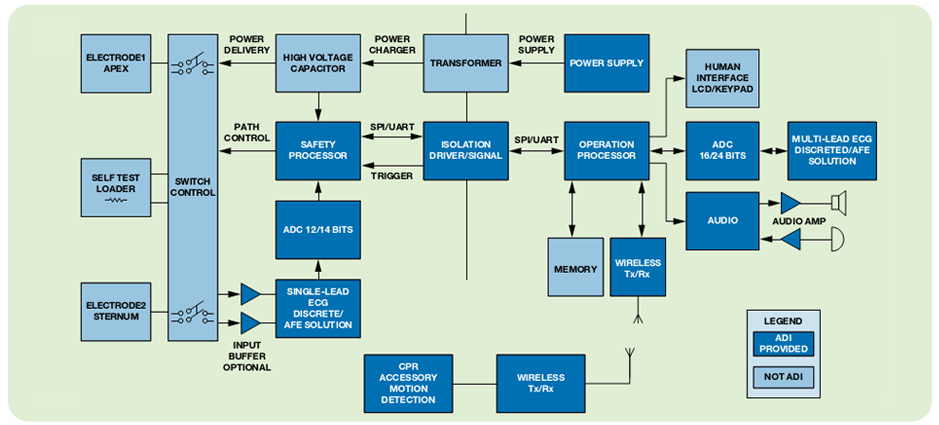
In under two decades, the portable, easy-to-use defibrillator has become of a routine part of the visual landscape and a welcome accessory on the wall; it is now mandated in many locales while establishments even boast that they have them available. The AED’s simplicity and effectiveness didn’t come easily but required a tight focus on what was needed, what was allowed, and what was achievable in this mission-critical mass-market application.
References:
- Philips Medical Systems, “Biphasic Defibrillation“
- Philips HeartStart FR3 Defibrillator, “Technical Reference Manual”
- Philips HeartStart OnSite, “Specifications”
- Philips HeartStart OnSite Defibrillator, “Owner’s Manual”
- Allied Market Research, “External Defibrillators Market by Product Type”
- IHS Report: “US Remote Cardiac Monitoring Market to Expand more than 25 Percent by 2016“
- Analog Devices, “AED Solutions”



Leave a Reply
You must be logged in to post a comment.