The transportation industry is responsible for a sizable chunk of energy consumption in the United States. Although electric vehicle adoption is underway, electric-powered submarines, ships, planes, and unmanned underwater vehicles (UUV) pose a harder transition, due to their requirements for both power and energy.
But that may not be the case for long. Engineers from Washington University’s McKelvey School of Engineering have developed a high-powered, direct borohydride fuel cell that trumps today’s tech by operating at double the voltage.
The fuel cell makes use of a pH-gradient microscale bipolar interface (PMBI), which according to Washington University, “could power a variety of transportation modes—including unmanned underwater vehicles, drones and eventually electric aircraft—at significantly lower cost.”
“The pH-gradient-enabled microscale bipolar interface is at the heart of this technology,” says Vijay Ramani, the Roma B. and Raymond H. Wittcoff Distinguished University Professor, and research team leader. “It allows us to run this fuel cell with liquid reactants and products in submersibles, in which neutral buoyancy is critical, while also letting us apply it in higher-power applications such as drone flight.”
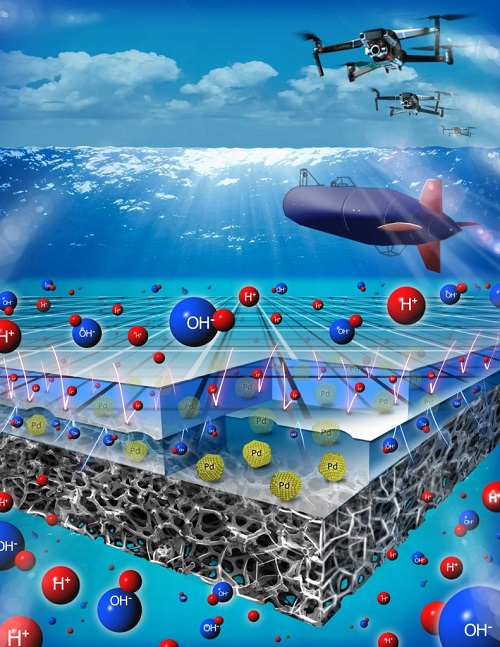
At one electrode sits an alkaline electrolyte, while an acidic electrolyte is used at the other electrode. When the two come into contact, they usually react quite quickly, but the team’s PMBI solves this issue. Thinner than a single strand of human hair, the PMBI prevents “the acid and alkali from mixing, forming a sharp pH gradient and enabling the successful operation of this system,” according to Washington University.
Shrihari Sankarasubramanian, a research scientist part of Ramani’s team, says, “Previous attempts to achieve this kind of acid-alkali separation were not able to synthesize and fully characterize the pH gradient across the PMBI. Using a novel electrode design in conjunction with electroanalytical techniques, we were able to unequivocally show that the acid and alkali remain separated.”
“Once the PBMI synthesized using our novel membranes was proven to work effectively, we optimized the fuel cell device and identified the best operating conditions to achieve a high-performance fuel cell,” adds Zhongyang Wang, a doctoral candidate in Ramani’s lab, and lead author of the research. “It has been a tremendously challenging and rewarding pathway to developing the new ion-exchange membranes that has enabled the PMBI.”
The researchers are currently seeking opportunities for commercialization by working with Washington University’s Office of Technology Management.
“This is a very promising technology, and we are now ready to move on to scaling it up for applications in both submersibles and drones,” Ramani says.
The PMBI advancement was reported in the article, “Efficient pH-gradient-enabled microscale bipolar interfaces in direct borohydride fuel cells,” published in Nature Energy.

