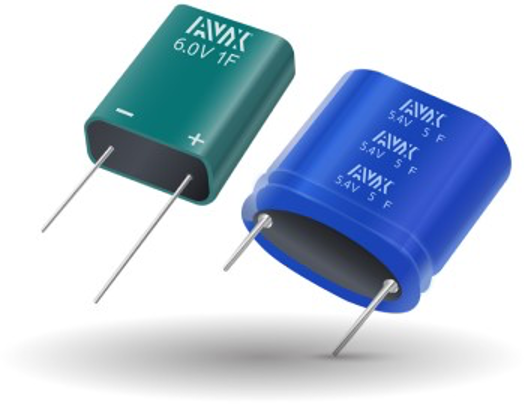
Thousands of Farads and power densities of thousands of watts per kilogram are among the chief benefits of supercapacitors (also called ultracapacitors). These devices operate in a fundamentally different way compared with conventional capacitors or batteries. Most supercapacitors commonly used today are double-layer capacitors which provide high power capabilities. Pseudo-capacitors are a less common form of supercapacitor and are typically optimized for long-term, lower power, backup power applications such as in solid state memories.
Standard supercapacitors (Image: AVX)
Supercapacitors are available in several formats including cylindrical, prismatic and pouch. In addition, they are offered as single cells or in prefabricated modules that deliver higher voltages and higher capacities. Some companies, such as Ioxus, have developed specially-designed supercapacitors optimized for use in modules. Supercapacitor modules can be standard off-the-shelf units or custom designs.
A theoretically ideal capacitor would provide only the specified capacitance. However, real devices are not ideal and include equivalent series inductance (ESL), equivalent series resistance (ESR), and insulation resistance (IR) in addition to capacitance. These various parasitic elements vary in magnitude between capacitor types as a result of the structure of the devices (e.g. planar versus wound, etc.) and the various energy storage mechanisms and materials employed.
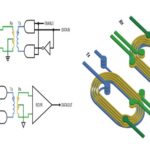
An equivalent circuit of an actual capacitor includes ESR (equivalent series resistance) and ESL (equivalent series inductance) (Image: Panasonic Industrial Devices
The lack of a dielectric layer is one of the key differences between supercapacitors and conventional capacitors. In supercapacitors, charge is physically stored on porous carbon electrodes (layers) separated by a very thin (several nanometers thick) electrolyte separator. On the electrode surfaces are Helmholtz layers where the electrons orient themselves opposite a layer of cations with equal and opposite charges on the electrolyte side of the interface. The resulting electric field has two layers of charges; hence it is called a double layer.
The electrodes for pseudocapacitors are typically made from materials such as MnO2 and RuO2. Those materials have the electrochemical signature of a capacitive electrode (linear dependence on current versus voltage curve) as well as exhibiting faradaic behavior. Additionally, the charge storage originates from electron-transfer mechanisms rather than accumulation of ions in the electrochemical double layer.
Charge storage principles of different capacitor types and their internal potential distribution (Image: Wikipedia)
The combination of the porosity of the layers together with the very thin separation between the layers results in a high packing density of the various layers and produces the high capacitance values and energy densities which supercapacitors are known to deliver. The thinness of the separation also results in a relatively low insulation resistance and the low voltage capabilities of supercapacitors, typically 2 to 3 volts.
The high power density of supercapacitors comes from a combination of the high energy density with the speed that the energy can be drained from the device. In a battery, the movement of charge carriers through an electrolyte is a relatively slow process that results in lower power densities compared with supercapacitors. The charge/discharge rates of supercapacitors are limited by current heating of the electrodes, not by the physical movement of charge carriers. That results in the high power densities of supercapacitors.
It’s the combination of the physical structure (small separation distances between the electrodes and the porosity of the electrodes) of supercapacitors along with the manner in which charge is stored in the electrodes that results in energy densities that are about one-tenth that of batteries along with power densities that can be two orders of magnitude greater than that of batteries.
Finally, it should be noted that while batteries and conventional capacitors are relatively mature technologies, supercapacitor technology continues to advance. In particular, advancements in electrode materials for supercapacitors continue to improve device performance. Graphene, carbon nanotubes, and carbon aerogels are being employed to close the performance gap between supercapacitors and batteries.
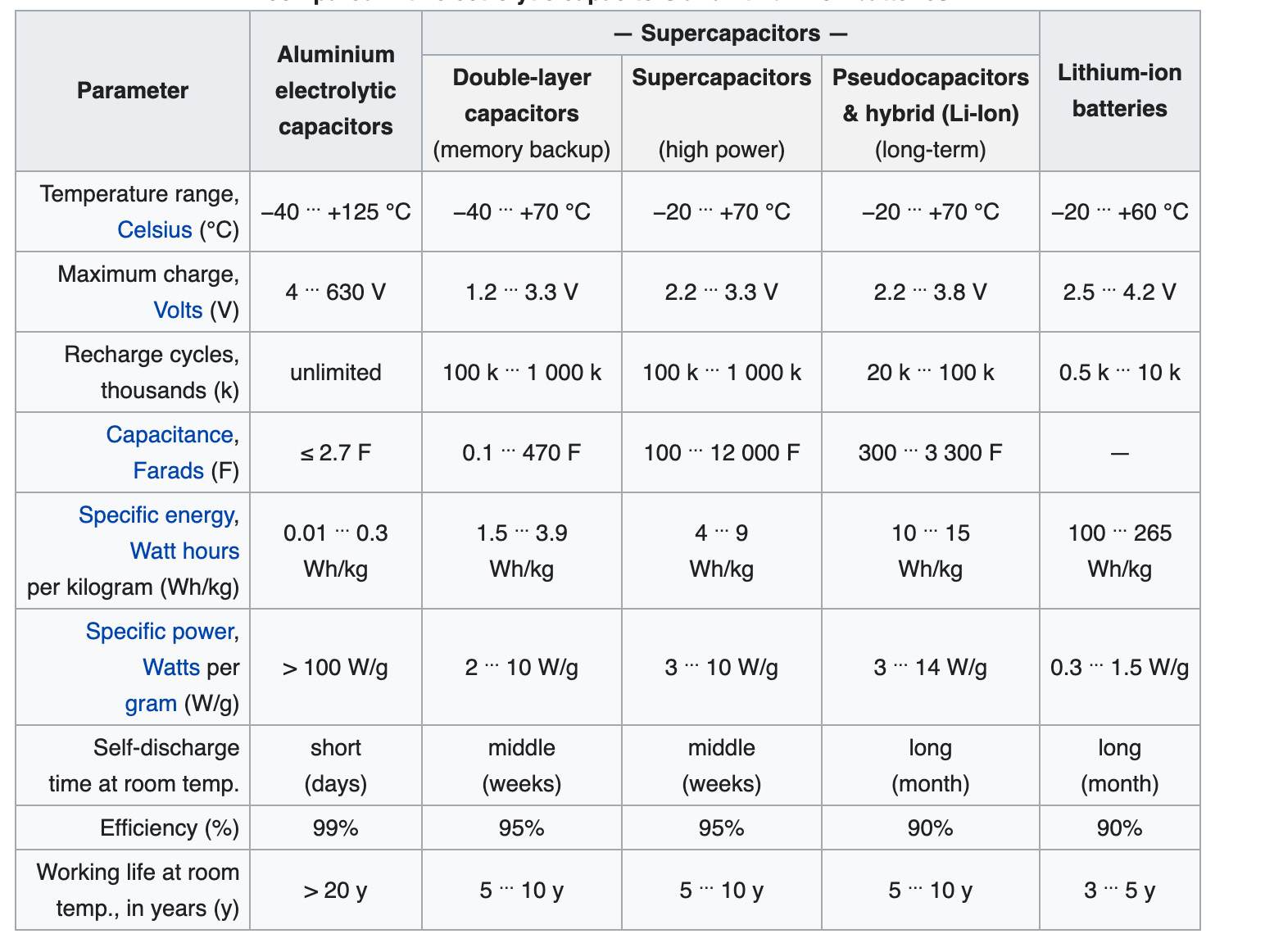
Another active area of electrode development is so-called hybrid capacitors with asymmetric electrodes, one of which exhibits mostly electrostatic and the other mostly electrochemical capacitance, such as lithium-ion capacitors. As a result of the dynamic nature of various supercapacitor technologies the chart below provides only a “snap shot” of current performance parameters, and the capabilities of various supercapacitor technologies will continue to improve in the future.
Current performance parameters, and the capabilities of various supercapacitor technologies (Image: Wikipedia
As seen above, the differing operating mechanism and construction details of supercapacitors lead to a different set of performance capabilities and specifications compared with both conventional capacitors and batteries. In addition to the differences outlined above, supercapacitors tend to have significantly higher internal resistance compared with aluminum electrolytic capacitors. That makes supercapacitors more susceptible to internal heat generation from ripple currents, potentially reducing device lifetimes. The next FAQ in this series, “Supercapacitor Specifications and IEC/EN 62391–1” will delve into this and other nuances of supercapacitor specifications.
References
Maxwell Technologies BOOSTCAP Ultracapacitors
Panasonic Fundamentals of Capacitors and Hybrid Capacitors
Wikipedia Supercapacitor
Ioxus, THiNCAP Cells