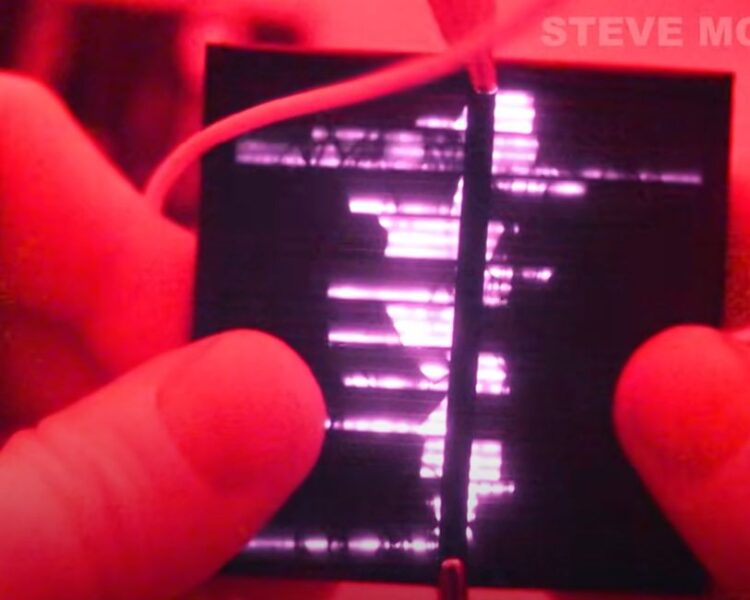
Perhaps thanks to YouTube, more people understand that light emitting diodes (LEDs) and solar cells may be thought of as mirror images of one another because the conversion of energy in each involves similar interactions of charge carriers. It’s easy to find YouTubers who have either created solar arrays from LEDs or rigged solar cells to emit light. Of course, LED-based solar panels are inefficient because LEDs aren’t optimized to convert light into charge carriers. Ditto for solar cells that emit light.

Actually, any ordinary diode will function as a solar cell or as and LED, either converting photons to charge carriers when exposed to light or generating photons when passing current. This doesn’t happen in ordinary diodes because their P-N junction is encased in a light=proof package. To see why, consider the reason a diode passes current in one direction but not the other. This diode action is often explained in terms of covalent bonds and the number of electrons in the atom’s outer shell.
The rule of eight or the Octet rule describes the tendency of atoms to have eight electrons in their valence shell. Eight electrons in this shell allow atoms to be stable and non-reactive. But atoms containing less than eight electrons in their final shell tend to bond with other atoms to get eight electrons there. An often-used example is fluorine with seven outer-shell electrons. It will readily bond with a another nearby fluorine atom to share one of the outer-shell electrons so each atom has eight outer electrons.
With this in mind, recall that silicon has four outer-shell electrons. So it tends to bond with multiple other silicon atoms three dimensionally to complete the final shell, resulting in a silicon crystal grown through covalent bonding. To make silicon a conductor, some of the silicon atoms are swapped out for an element with extra electrons (such as phosphorus) or fewer electrons (such as boron). This creates charge carriers, either electrons or holes into which nearby electrons jump.
A diode, of course, contains one piece of N-type silicon with extra electrons and one piece of P-type silicon with extra holes. At the junction of the two materials, electrons of the N-type silicon diffuse a bit into the P-type silicon to fill the holes there, building up negative charge. Similarly, spots the electrons have left in the N-type material create a buildup of positive charge. In the absence of an external voltage, there’s an equilibrium in the so-called depletion zone at the junction of the N and P-type material.
Connecting the poitive terminal of a battery to the N-type material causes freely moving negatively charged electrons to move toward the positive battery node because opposites attract. Simultaneously, holes in the P-type material get pulled toward the negative battery terminal. In this scenario, the depletion zone grows larger and no current can flow.
Reversing the polarity of the battery pushes electrons into the N-type material and holes into the P-type material, toward the diode junction. The depletion zone shrinks, and a sufficiently large voltage makes electrons jump across the smaller zone.
A point to note is that when an electron jumps across the depletion zone and enters a hole, it goes from a high energy state to a low one. When an electron does that, it emits a photon. This action, of course, is what makes LEDs possible. The further the electron drops, the more energetic the photon released. There is a link between the energy of a photon and its color. So the more energy a photon has, the higher the light frequency.
Thus if we cracked open an ordinary silicon diode and turned it on, it would emit light in the infrared range. Real LEDs use materials with electrons that have bigger energy drops. Gallium arsenide-phosphide LEDs can emit red, orange and yellow. Gallium nitride devices have a bigger bandgap that yields blue light.
Now suppose light shines on the P-N junction. This action creates photons impinging on the junction. If the photons are energetic enough, they can cause an electron to leave a shell and leave a hole behind. In other words, the photon creates an electron-hole pair. Because there is an electric field in the depletion zone, the electron is pushed into the N-type material while the hole is pushed into the P-type material. This action generates a voltage across the diode, as in a photocell.
One reason LEDs don’t make good photovoltaic cells is that their bandgaps are tuned for emitting light, not reacting to it. A relationship called the Shockley-Queisser limit is the maximum efficiency of solar cells based on the principle of detailed balance (basically, that all photons get absorbed above the band gap). It places the maximum solar conversion efficiency at 33.7% for a single-junction solar cell with a band gap of 1.4 eV. If the band gap is too high, most photons will not cause a photovoltaic effect; if it is too low, most photons will have more energy than necessary to excite electrons across the band gap, and the rest of the energy will be wasted.
 In contrast, the bandgap of LEDs is set depending on the desired wavelength of light they emit. Visible red-emitting LEDs have a band gap of about 1.9 eV, yellow 2.1 eV, green 2.4 eV, and blue 2.64 eV. Consequently, LEDs used as photovoltaic cells typically generate low currents. That’s why many of the YouTubers who craft LED-based photovoltaic arrays feed the array output to what’s called a joule thief circuit.
In contrast, the bandgap of LEDs is set depending on the desired wavelength of light they emit. Visible red-emitting LEDs have a band gap of about 1.9 eV, yellow 2.1 eV, green 2.4 eV, and blue 2.64 eV. Consequently, LEDs used as photovoltaic cells typically generate low currents. That’s why many of the YouTubers who craft LED-based photovoltaic arrays feed the array output to what’s called a joule thief circuit.
A joule thief is a voltage booster circuit which converts a constant low voltage input into pulses of a higher voltage. This circuit typically uses a ferrite toroid core wrapped with two wires. One comes from the positive terminal of the solar cell. The other coil drives the base of a transistor, typically through a resistor also connected to the solar cell. Current passing through the wires of the toroid creates a magnetic field that switches off the transistor and cuts power to the toroid. Consequently, the collapsing magnetic field is converted to electrical energy to drive a load (which typically includes a capacitor to level out the pulse energy). When the pulse ends, the transistor switches back on to again create a magnetic field.