By designing a solid electrolyte that is rigid on one side and soft on the other, researchers have fabricated a lithium-metal battery that completely suppresses dendrite formation—a major safety hazard that can cause fires and shorten battery lifetime. This design also overcomes a tradeoff that is typically present in these batteries, by simultaneously eliminating dendrite growth and reducing the resistance at the electrode/electrolyte interface. Typical methods cannot achieve both of these goals at the same time.
The researchers, from Profs. Yu-Guo Guo and Li-Jun Wan’s groups of the Chinese Academy of Sciences and the University of Chinese Academy of Sciences, have published a paper on the new electrolyte in a recent issue of the Journal of the American Chemical Society.
“We have proposed an asymmetric solid electrolyte, which can concurrently meet the requirements of a dendrite-free lithium anode and a low interface resistance in solid batteries,” Guo told Phys.org.
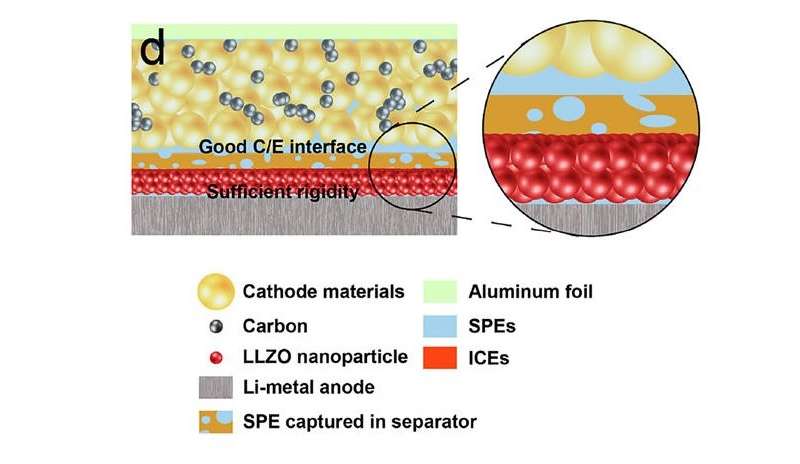
As the researchers explain, the tradeoff in lithium batteries occurs because the lithium anode and the cathode have requirements that are inherently contradictory. While the anode requires a rigid electrolyte to block dendrite growth, it is difficult for a rigid electrolyte to maintain sufficient contact with the solid cathode, which creates a highly resistive cathode/electrolyte interface.
To address this problem, the researchers designed an asymmetrical solid electrolyte, in which each side has a different type of surface. The side facing the anode is a rigid ceramic material that presses against the lithium anode to discourage dendrite growth. On the other hand, the side facing the cathode is made of a soft polymer, which allows for a strong interfacial connection with the cathode. The entire electrolyte is also very thin, at just under 36 micrometers.
In tests, the researchers compared batteries with the new electrolyte to those with a conventional electrolyte. After 1750 hours of cycling, they found that the batteries with the conventional electrolyte exhibited rough morphologies indicative of dendrite formation, while those with the new electrolyte showed no morphological changes even after 3200 hours of cycling, indicating that dendrite growth was effectively eliminated.
Going forward, the researchers expect that the dendrite-free lithium batteries will lead to energy-storage systems that combine the high energy and power densities of lithium batteries with improved safety and longer lifetimes due to eliminating dendrite growth.
“We plan to design pouch cells with this asymmetric solid electrolyte for attaining a high energy density in solid batteries,” Guo said.