The personal defibrillator combines sophisticated electronics with precise, adaptive control of the high-energy pulses which can re-start the heart’s beating but do so without shocking the patient to death.
You’ve seen those life-saving defibrillators in offices, malls, and even homes, quietly at the ready for immediate use. When the Philips Medical Systems division of Royal Philips Electronics introduced their HeartStart defibrillator in 2002, it was the first low-cost, mass-market unit designed for ordinary, untrained users.
An AED requires much more than just application of a high voltage to the patient’s chest, and dramatically shouting “clear!” This FAQ will look at the underlying medical parameters for the first such device and how this very successful, life-saving unit was designed.
Q: What is the “personal” defibrillator?
A: This unit is formally known as an automated external defibrillator (AED) to distinguish it from the implanted pacemaker. The first introduced version was small (a little bigger than a large hardcover book), lightweight (2.1 kg/4.6 pounds), and featured extreme ease of use with user prompts provided by a soothing voice (Figure 1). Defibrillators “deliver therapeutic shock to a patient’s heart in life-threatening conditions such as ventricular fibrillation, cardiac arrhythmia, and pulseless ventricular tachycardia. The defibrillation procedure encompasses delivery of an electric shock to the heart, which depolarizes heart muscles and restores its normal electric impulse.”
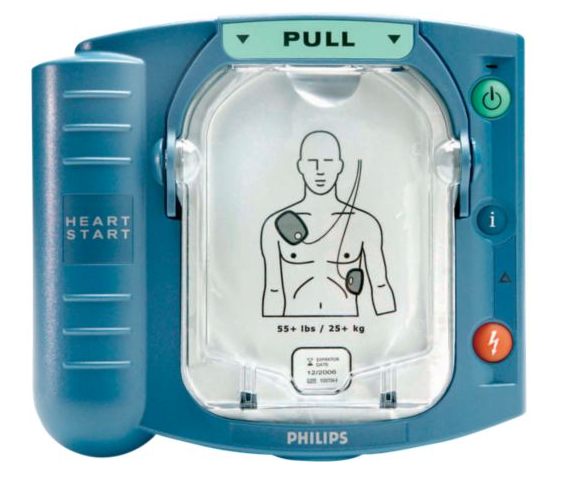
Q: Was it a successful product?
A: Absolutely. It was soon either mandated or voluntarily installed in tens of thousands of public locations and private homes. Specific numbers are not available for competitive reasons, but many market-research organizations try to provide estimates. For example, according to Allied Market Research (Reference 5), the AED market “was valued at $4.345 billion in 2016 and is expected to grow to $7.512 million by 2023, registering a CAGR (compound annual growth rate) of 7.8% during the forecast period 2017 – 2023.” [Don’t ask how they project market size five years into the future with precision to four significant figures, when it seems that an error bound of perhaps ±10 to ±20% makes more sense here.) At an average selling price of $1500 – $2000, that is a lot of units.
Q: How did the design process begin?
A: The initial AED design began with a team at Heartstream, Inc, (US Patent 5,735,879, April 7, 1998), prior to the company’s acquisition by Hewlett-Packard/Agilent (1998), who subsequently sold it to Philips Medical Systems (2001). The product had to meet a complex maze of constraints with respect to size, weight, performance, regulatory mandates, and cost (under $2000 retail).
Q: What did the design require?
A: It required a significant amount of innovative circuitry based largely on standard components and a custom ASIC processor (Figure 2), to provide its non-intimidating appearance and operation. Despite the inherently complex functionality of defibrillation, the designers did not want a GUI (graphical user interface), multiline text display, or touchscreen for user direction and guidance — they chose to use just a voice for user guidance.
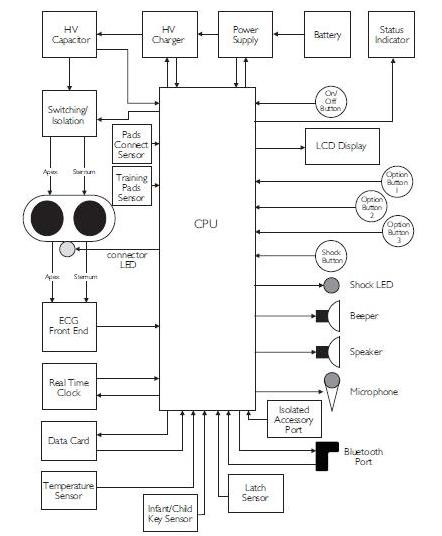
Q: How did they size and shape the enclosure?
A: Obviously, size is critical. The unit had to be large enough for the required electronics and internal high-voltage isolation and batteries, but not too big (bulky, awkward, unattractive) or too small (too easily knocked away or overlooked). In a conversation with one of the designers several years ago, I was told that most of the “rough” enclosure sizing and shaping was done using plain, brown corrugated cardboard which could easily be cut, trimmed, adjusted, and modified. Only when they were pretty close to a likely prototype form factor did they get tooling for a formal enclosure.
Q: What were the primary challenges the design team faced?
A: The design addresses three traditional analog challenges: 1) generation of carefully shaped high-voltage/current pulses from a low-voltage, small-capacity battery pack; 2) need for analog inputs to make sensitive measurements circuit at the contact points of these; and 3) the need for sophisticated battery/power-management algorithms for devices having long idle periods followed by sudden, unanticipated operating cycles.
Q: What does the defibrillator waveform look like?
A: Successful defibrillation depends on multiple factors: the voltage of the delivered waveform, the energy (joules) of that waveform, and the shape of the waveform are among the many variables.
Q: Historically, what was the first defibrillator waveform?
A: In the early years of defibrillators (in the 1940s), the waveform was nothing more than the basic 50/60-Hz AC line, stepped up by a factor of 5× or 10× via a standard transformer. Effectiveness was minimal while the risk to the cardiac patient was quite high. Work was done in the 1950s on more complex defibrillation protocols, with the goals of having battery-powered units and providing a more effective, yet safer, waveform.
Q: What waveform was eventually devised?
A: The most-effective tested and approved resuscitation pulse is the biphasic truncated exponential (BTE) waveform, which reaches up to as high as +1750 V and down to about -500 V. Its period ranges between 5 and 20 msec, with currents reaching 20 A, corresponding to total energy of about 150 J.
Part 2 of this FAQ will explore the actual design and implementation of this first AED.
References:
- Philips Medical Systems, “Biphasic Defibrillation“
- Philips HeartStart FR3 Defibrillator, “Technical Reference Manual”
- Philips HeartStart OnSite, “Specifications”
- Philips HeartStart OnSite Defibrillator, “Owner’s Manual”
- Allied Market Research, “External Defibrillators Market by Product Type”
- IHS Report: “US Remote Cardiac Monitoring Market to Expand more than 25 Percent by 2016“
- Analog Devices, “AED Solutions”




Leave a Reply
You must be logged in to post a comment.